Abstract
Introduction: The VENARES study confirmed that venetoclax in monotherapy (VenM) or combined with rituximab (VenR) was effective in heavily pretreated CLL patients (pts) (ORR at 9 months: 84.3%); and safety was consistent with prior experiences. Here we present an additional analysis of efficacy and safety comparing subgroups.
Methods: This is non-interventional retrospective, multicenter, post-marketing, observational study where data from adult CLL pts treated with Ven at least 9 months before were included. According to local label, pts were eligible for VenM, treatment continued until disease progression or no longer tolerated, or VenR treatment, venetoclax administered for up to 2 years plus rituximab for the first 6 months. Pts were reviewed until the date of last follow-up or death.
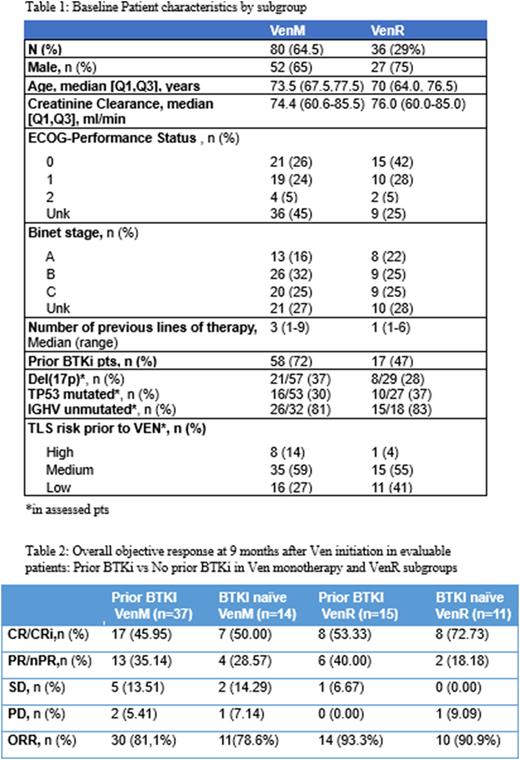
Results: One hundred and twenty-four out of 125 eligible pts were finally assessed for efficacy. VenM in 80 pts (64.5%) and VenR in 36 pts (29%). Ven with obinutuzumab in 5 pts (4%) and with other drugs in 3 pts (2.4%). Patient characteristics by subgroup are summarized in Table 1.
Eighty-three of 125 pts were evaluable for the primary objective: overall response rate (ORR) at 9 mo was 84.3% (70 pts): CR/CRi in 44 (53%) and PR/nPR in 26 pts (31.3%). By subgroups, ORR at 9 mo was 80.4% (41 of 51 pts) in VenM pts, with 47% of CR/CRi, and 33.3% PR/nPR; and 92.3.% (24 of 26 pts) in VenR pts, with 61.5% CR/CRi, and 30.8% PR/nPR.
ORR at 9 mo were 81% for prior bruton tyrosine kinase (BTKi) treated VenM pts and 78.6% for BTKi naïve VenM pts; and 93.3% for prior BTKi VenR pts and 90.9% for BTKi naïve VenR pts (Table 2).
Sixty-four of 83 evaluable pts had reached 400 mg of Ven. ORR at 9 mo were 85.94% in pts who had received 400 mg and 78.95% in the rest. In VenM pts, ORR at 9 mo were 80% for 400 mg and 81.3% for the rest. In VenR pts, ORR at 9 mo was 92% for 400 mg, all but one pts reached the full dose.
The median PFS was not reached at the time of the analysis (18-May-2022) in either subgroup. In VenM pts, PFS at 18 mo was 78.7% (95% CI, 67.4- 86.5) and at 24 mo 72.7% (95% CI, 55.3-84.2). In VenR pts, PFS at 18 mo was 89.1% (95% CI, 57.9- 97.6) and not able to be estimated at 24 mo.
In assessed pts, best undetectable MRD was reached in 13 pts (43.3%). uMRD was more common in VenR pts (83.3%, 5 of 6 pts) than in VenM (33.3%, 8 of 24 pts).
Sixty-seven pts (53.6%) experienced at least 1 specific AE and 14 pts at least 1 specific SAE both related to Ven. In 34 pts, the treatment was withdrawn, 13 of which were due to AE, all of them in the VenM subgroup.
Forty-four of 80 pts (55%) and 17 of 36 (47.2%) pts in VenM and VenR subgroups reported at least 1 specific AE related to Ven. 22 pts (27.5%) and 7 pts (19.4%) had neutropenia G3/4 in VenM and VenR, respectively. 6 pts (7.5%) and 3 (8.3%) had febrile neutropenia in VenM and VenR. Seven pts (8.75%) and 0 pts had a serious infection in VenM and VenR. 3 pts (3.75%) and 1(2.78%) had a TLS event in VenM and VenR; there was only 1 pt with clinical TLS non-related to Ven in the VenM subgroup. Two (2.5%) and 0 pts presented a Richter transformation in VenM and VenR, respectively.
In pts with known TLS risk during Ven ramp-up, hospitalized pts were: 20% (8/40) with high risk, 62.5% (25/40) medium and 17.5% (7/40) low risk in VenM. In VenR subgroup, 6.2% (1/16) with high risk, 62.5% (10/16) medium and 31.3% (5/16) low risk.
Conclusions: Updated subgroup analysis showed that treated pts with VenR fixed treatment duration achieved higher clinical responses compared to VenM (ORR at 9 mo 92.3% vs 80%) however VenR pts had been less heavily pretreated. ORR at 9 mo was maintained in both BTKi exposed and BTKi naïve pts in the two subgroups, and BTKi naïve pts had a higher percentage of CR/CRi compared to BTKi exposed pts, especially in VenR subgroup. Pts that achieved full dose of Ven (400mg) achieved higher clinical responses. No new safety events were detected.
Disclosures
Ferra:Janssen, Roche, Gilead, Takeda, Abbvie: Consultancy, Other: Medical meetings funding. Terol:Janssen, Abbvie, Roche, Takeda, Astra-Zeneca: Consultancy. Moreno:Abbvie, Janssen, AstraZeneca, Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen, Abbvie: Research Funding. Osorio:Janssen, Abbvie, Roche: Consultancy. De la Cruz:Beigene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eusa Pharma: Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. de la Serna:AbbVie, AstraZeneca, Beigene, Gilead, GSK, Janssen, Jazzpharma, Novartis, Roche: Consultancy; Abbvie, AstraZeneca, Roche: Research Funding; Abbvie, AstraZeneca, Roche: Speakers Bureau. Arguiñano:Abbvie: Honoraria; Sanofi: Honoraria; BMS-Celgene: Honoraria; Janssen: Honoraria; Amgen: Honoraria; Sandoz: Speakers Bureau; Novartis: Honoraria; AstraZeneca: Honoraria; GSK: Honoraria. Loscertales:Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Speakers Bureau; Lilly: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Speakers Bureau; Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees. García:Janssen, Roche, Gilead, Celgene, Abbvie: Other: medical meetings funding; Janssen, Abbvie: Research Funding; Janssen, Roche, Gilead, Celgene: Consultancy. Muntañola Prat:Abbvie: Honoraria, Speakers Bureau; AstraZeneca: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Roche: Honoraria, Speakers Bureau. Perez Persona:Celgene/BMS, Amgen, Janssen, GSK: Consultancy; Celgene/BMS, Amgen, Abbvie. Takeda, Sanofi, Astra-Zeneca: Speakers Bureau; Roche, Celgene/BMS, Amgen, Janssen, Abbvie, Jazz Pharmaceutical: Other: medical meetings funding. Pérez-Encinas:Janssen: Consultancy. Caballero:Celgene, Janssen, Novartis, Abbvie: Speakers Bureau; Celgene, Janssen, Amgen: Consultancy. Ruiz-Zorrilla:Abbvie: Current Employment. Moreno:Abbvie: Current Employment. Baltasar:Abbvie, Janssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal